14. Solutions
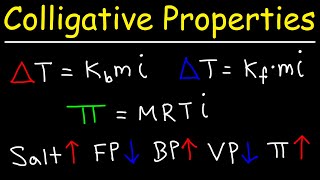
Vapor Pressure Lowering (Raoult's Law)
Problem 71
Textbook Question
Textbook QuestionCalculate the vapor pressure of a solution containing 24.5 g of glycerin (C3H8O3) in 135 mL of water at 30.0 °C. The vapor pressure of pure water at this temperature is 31.8 torr. Assume that glycerin is not volatile and dissolves molecularly (i.e., it is not ionic), and use a density of 1.00 g>mL for the water.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
2865
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos