14. Solutions
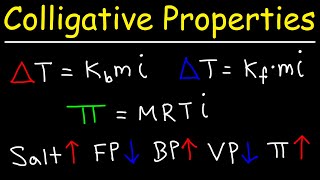
Vapor Pressure Lowering (Raoult's Law)
Problem 116
Textbook Question
Textbook QuestionThe density of a 0.438 M solution of potassium chromate (K2CrO4) at 298 K is 1.063 g>mL. Calculate the vapor pressure of water above the solution. The vapor pressure of pure water at this temperature is 0.0313 atm. (Assume complete dissociation of the solute.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1272
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos