15. Chemical Kinetics
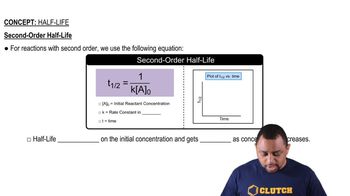
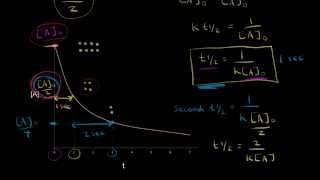
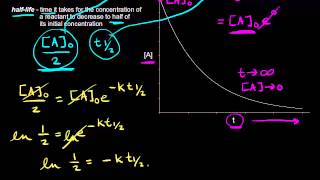
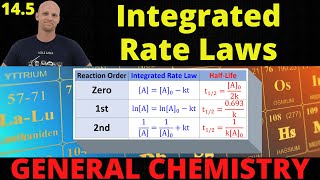
Half-Life
Problem 83
Textbook Question
Textbook QuestionThe tabulated data were collected for this reaction at 500 °C: CH3CN(g)¡CH3NC( g) Time (h) [Ch3Cn] (M) 0.0 1.000 5.0 0.794 10.0 0.631 15.0 0.501 20.0 0.398 25.0 0.316 b. What is the half-life for this reaction (at the initial concentration)?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
387
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos