7. Gases
The Ideal Gas Law Derivations
Problem 58
Textbook Question
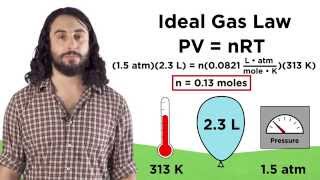
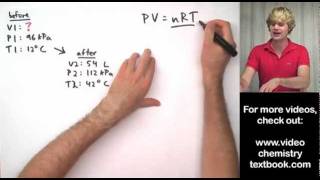
Textbook QuestionA small cylinder of helium gas used for filling balloons has a volume of 2.30 L and a pressure of 13,800 kPa at 25 °C. How many balloons can you fill if each one has a volume of 1.5 L and a pressure of 1.25 atm at 25 °C?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1579
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos