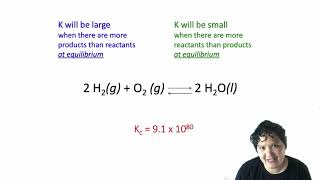16. Chemical Equilibrium
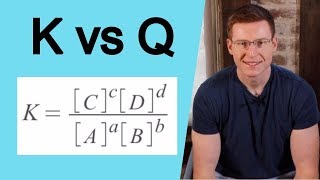
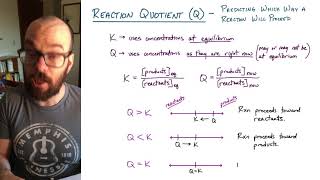
Reaction Quotient
Problem 84
Textbook Question
Textbook QuestionAt 900 C, Kc = 0.0108 for the reaction CaCO31s2 Δ CaO1s2 + CO21g2 A mixture of CaCO3, CaO, and CO2 is placed in a 10.0-L vessel at 900 C. For the following mixtures, will the amount of CaCO3 increase, decrease, or remain the same as the system approaches equilibrium? (c) 30.5 g CaCO3, 25.5 g CaO, and 6.48 g CO2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
627
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos