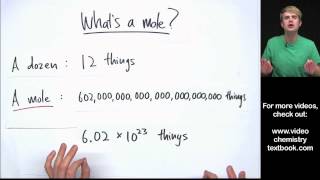2. Atoms & Elements
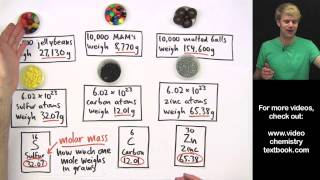
Mole Concept
Problem 90b
Textbook Question
Textbook QuestionNatural gas is a mixture of hydrocarbons, primarily methane 1CH42 and ethane 1C2H62. A typical mixture might have Xmethane = 0.915 and Xethane = 0.085. Let's assume that we have a 15.50 g sample of natural gas in a volume of 15.00 L at a temperature of 20.00 °C. (a) How many total moles of gas are in the sample?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
398
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos