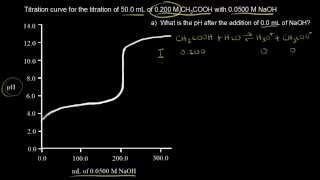
18. Aqueous Equilibrium
Titrations: Weak Acid-Strong Base
Problem 106
Textbook Question
Textbook Question(a) By titration, 15.0 mL of 0.1008 M sodium hydroxide is needed to neutralize a 0.2053-g sample of a weak acid. What is the molar mass of the acid if it is monoprotic?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1223
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos