2. Atoms & Elements
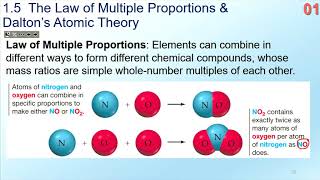
Law of Multiple Proportions

Get help from an AI Tutor
Ask a question to get started.
Problem 14a
Textbook Question
Textbook QuestionIn a series of experiments, a chemist prepared three different compounds that contain only iodine and fluorine and determined the mass of each element in each compound: Compound Mass of Iodine (g) Mass of Fluorine (g) 1 4.75 3.56 2 7.64 3.43 3 9.41 9.86 (b) How do the numbers in part (a) support the atomic theory?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
450
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos