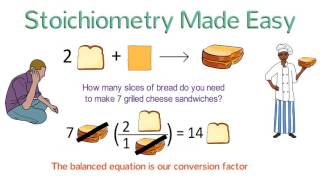
3. Chemical Reactions
Stoichiometry
Problem 70b
Textbook Question
Textbook QuestionDetonation of nitroglycerin proceeds as follows: 4 C3H5N3O91l2¡ 12 CO21g2 + 6 N21g2 + O21g2 + 10 H2O1g2 (a) If a sample containing 2.00 mL of nitroglycerin 1density = 1.592 g>mL2 is detonated, how many moles of gas are produced?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1004
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos