20. Electrochemistry
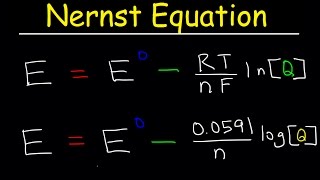
Cell Potential: The Nernst Equation
Problem 73
Textbook Question
Textbook QuestionA voltaic cell employs the following redox reaction: Sn2+(aq) + Mn(s) ¡ Sn(s) + Mn2+(aq) Calculate the cell potential at 25 °C under each set of conditions. c. [Sn2+] = 2.00 M; [Mn2+] = 0.0100 M
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
3405
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos








![19.5 How to Calculate Nonstandard Cell Potential [Nernst Equation] | General Chemistry](https://img.youtube.com/vi/Ma0TC3V9bdI/mqdefault.jpg)