7. Gases
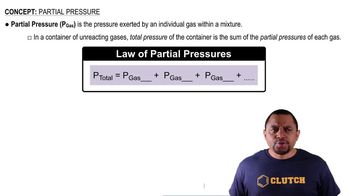
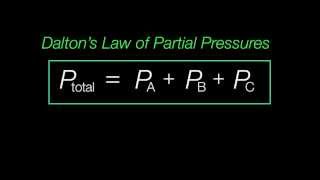
Partial Pressure
Problem 69
Textbook Question
Textbook QuestionA rigid vessel containing a 3:1 mol ratio of carbon dioxide and water vapor is held at 200 °C where it has a total pressure of 202.7 kPa. If the vessel is cooled to 10 °C so that all of the water vapor condenses, what is the pressure of carbon dioxide? Neglect the volume of the liquid water that forms on cooling.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
980
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos