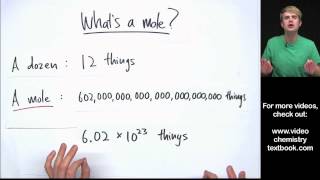2. Atoms & Elements
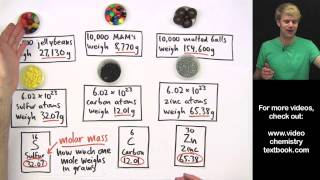
Mole Concept
Problem 82a
Textbook Question
Textbook QuestionDetermine the number of moles of oxygen atoms in each sample. a. 4.88 mol H2O2 b. 2.15 mol N2O c. 0.0237 mol H2CO3 d. 24.1 mol CO2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3464
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos