18. Aqueous Equilibrium
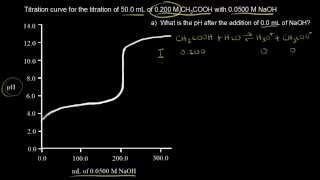
Titrations: Weak Acid-Strong Base
Problem 84
Textbook Question
Textbook QuestionThe distinctive odor of vinegar is due to acetic acid, CH3COOH, which reacts with sodium hydroxide according to: CH3COOH1aq2 + NaOH1aq2¡ H2O1l2 + NaCH3COO1aq2 If 3.45 mL of vinegar needs 42.5 mL of 0.115 M NaOH to reach the equivalence point in a titration, how many grams of acetic acid are in a 1.00-qt sample of this vinegar?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1131
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos