7. Gases
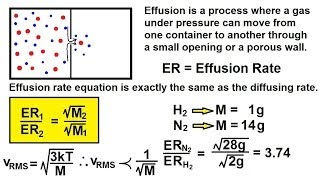
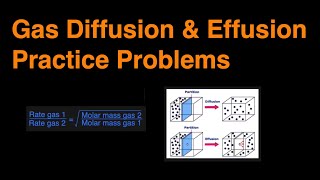
Effusion
Problem 88a
Textbook Question
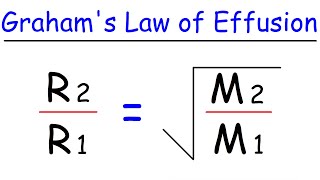
Textbook QuestionA gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 31 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas. (Remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 L; in other words, rate is the amount that diffuses over the time it takes to diffuse.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1704
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos