8. Thermochemistry
Hess's Law
Problem 81
Textbook Question
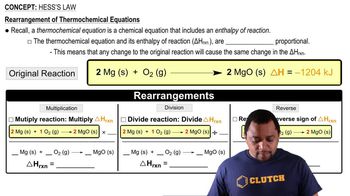
Textbook QuestionCalculate ΔHrxn for the reaction: 5 C(s) + 6 H2( g)¡C5H12(l ) Use the following reactions and given ΔH's: C5H12(l ) + 8 O2( g)¡5 CO2( g) + 6 H2O( g) ΔH = -3244.8 kJ C(s) + O2( g)¡CO2( g) ΔH = -393.5 kJ 2 H2( g) + O2( g)¡2 H2O( g) ΔH = -483.5 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
2585
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos