3. Chemical Reactions
Percent Yield
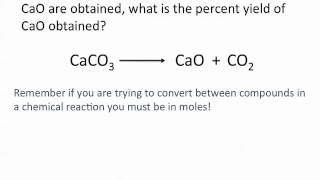
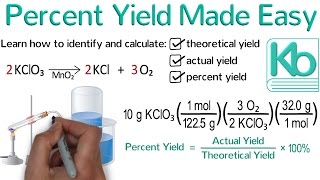
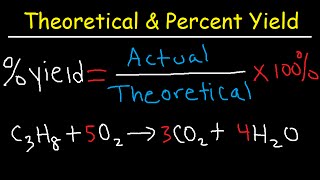
Problem 81
Textbook Question
Textbook QuestionCisplatin [Pt(NH3)2Cl2], a compound used in cancer treat-ment, is prepared by reaction of ammonia with potassium tetrachloroplatinate: K2PtCl4 + 2 NH3 ----> 2 KCl + Pt(NH3)2Cl2 How many grams of cisplatin are formed from 55.8 g of K2PtCl4 and 35.6 g of NH3 if the reaction takes place in 95% yield based on the limiting reactant?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1603
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos