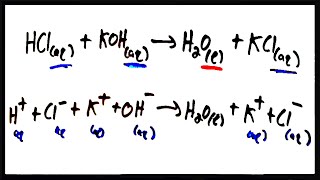6. Chemical Quantities & Aqueous Reactions
Complete Ionic Equations
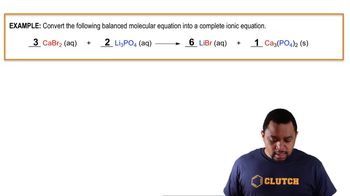
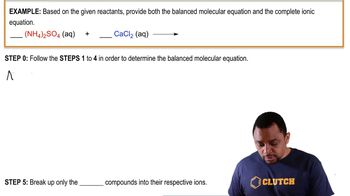
Problem 82
Textbook Question
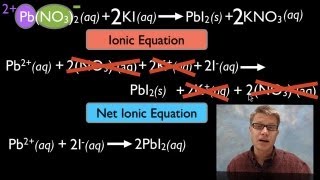
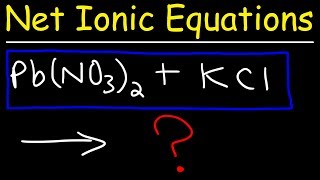
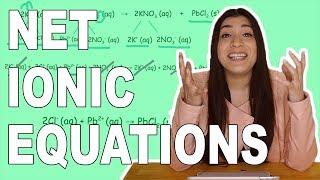
Textbook QuestionLead(II) ions can be removed from solution by precipitation with sulfate ions. Suppose that a solution contains lead(II) nitrate. Write complete ionic and net ionic equations for the reaction of aqueous lead(II) nitrate with aqueous potassium sulfate to form solid lead(II) sulfate and aqueous potassium nitrate.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1254
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos