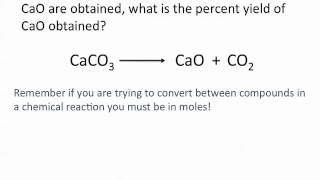
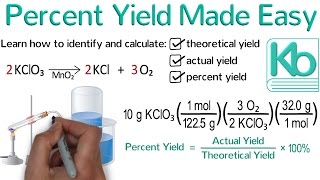
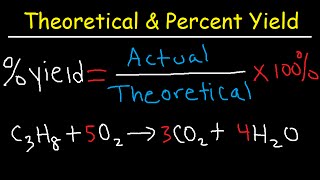
3. Chemical Reactions
Percent Yield
Problem 85
Textbook Question
Textbook QuestionHydrogen sulfide is an impurity in natural gas that must be removed. One common removal method is called the Claus process, which relies on the reaction: 8 H2S1g2 + 4 O21g2¡S81l2 + 8 H2O1g2 Under optimal conditions the Claus process gives 98% yield of S8 from H2S. If you started with 30.0 g of H2S and 50.0 g of O2, how many grams of S8 would be produced, assuming 98% yield?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1667
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos