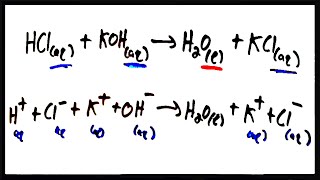6. Chemical Quantities & Aqueous Reactions
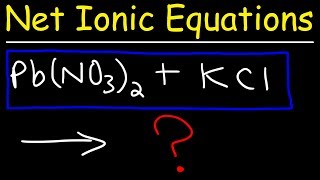
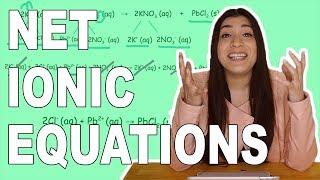
Complete Ionic Equations
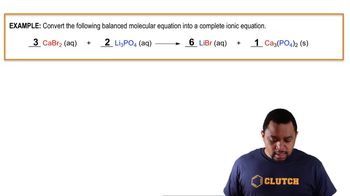
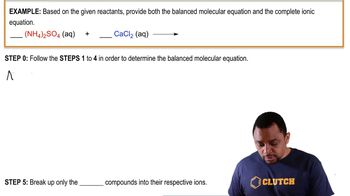
Problem 102
Textbook Question
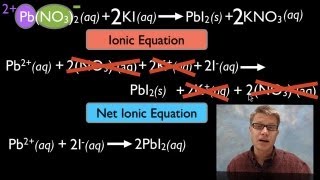
Textbook QuestionCitric acid, C6H8O7, is a triprotic acid. It occurs naturally in citrus fruits like lemons and has applications in food flavouring and preservatives. A solution containing an unknown concentration of the acid is titrated with KOH. It requires 23.20 mL of 0.500 M KOH solution to titrate all three acidic protons in 100.00 mL of the citric acid solution. Write a balanced net ionic equation for the neutralization reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
653
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos