13. Liquids, Solids & Intermolecular Forces
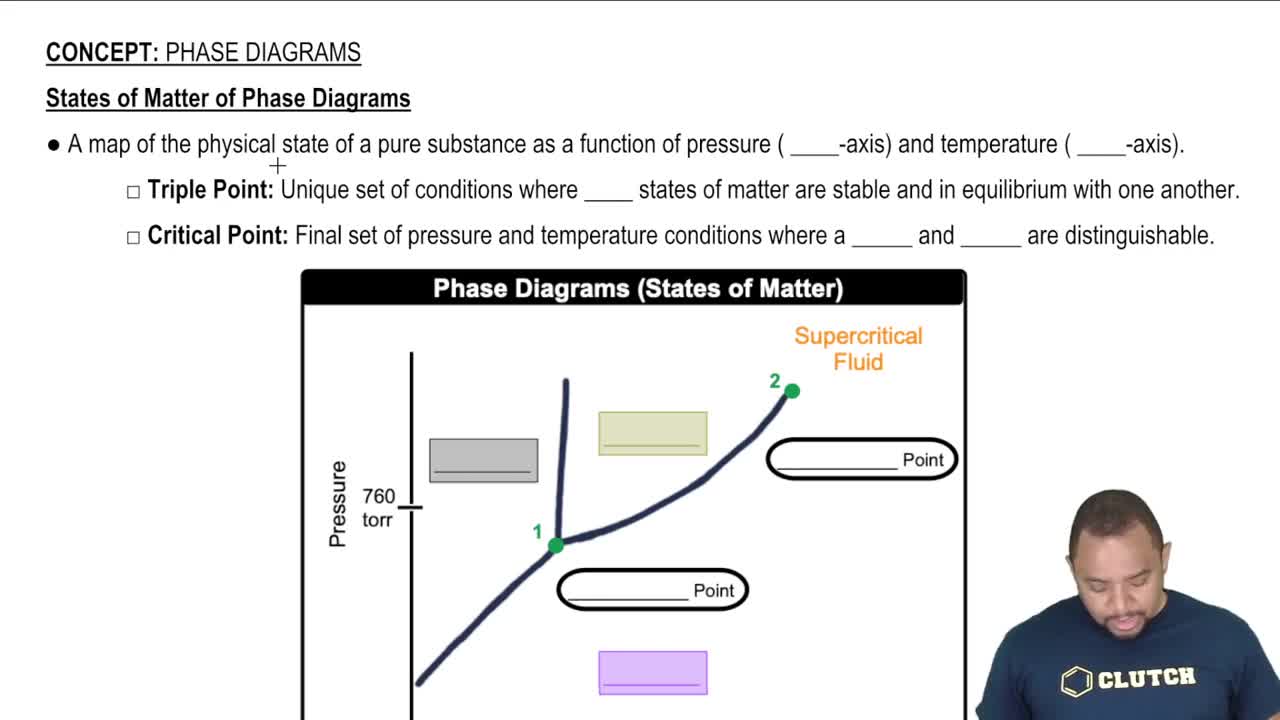
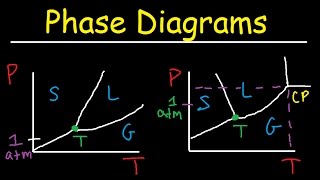
Phase Diagrams
Problem 77a
Textbook Question
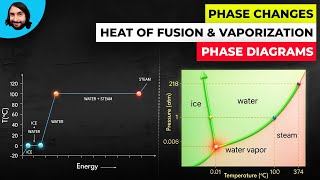
Textbook QuestionSubstance X has a vapor pressure of 100 mm Hg at its triple point (48 °C). When 1 mol of X is heated at 1 atm pres- sure with a constant rate of heat input, the following heating curve is obtained: (b) For each of the following, choose which phase of X (solid, liquid, or gas) fits the description. (iii) Has the greatest specific heat

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
465
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos