2. Atoms & Elements
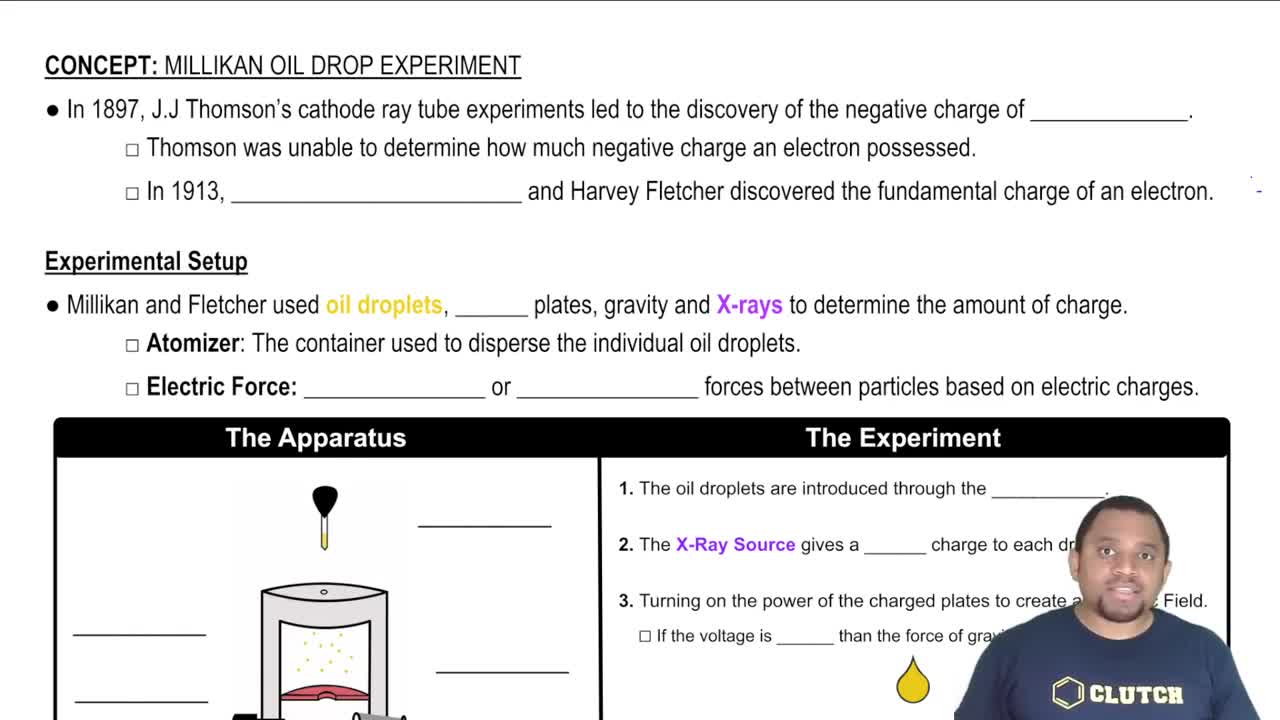
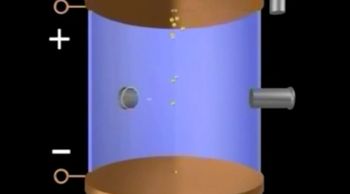
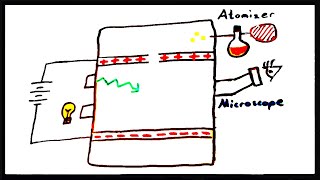
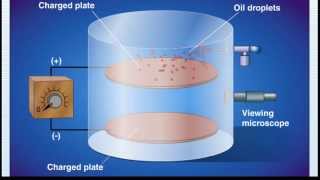
Millikan Oil Drop Experiment
Problem 87c
Textbook Question
Textbook QuestionSuppose a scientist repeats the Millikan oil-drop experiment but reports the charges on the drops using an unusual (and imaginary) unit called the warmomb (wa). The scientist obtains the following data for four of the drops: Droplet Calculated Charge (wa) A 3.84 * 10-8 B 4.80 * 10-8 C 2.88 * 10-8 D 8.64 * 10-8 (b) From these data, what is the best choice for the charge of the electron in warmombs?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
611
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos