18. Aqueous Equilibrium
Intro to Acid-Base Titration Curves

Get help from an AI Tutor
Ask a question to get started.
Problem 34
Textbook Question
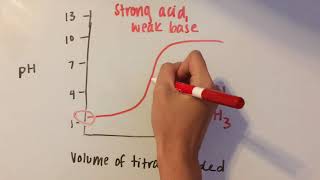
Textbook QuestionCompare the titration of a strong, monoprotic acid with a strong base to the titration of a weak, monoprotic acid with a strong base. Assume the strong and weak acid solutions initially have the same concentrations. Indicate whether the following statements are true or false. (a) More base is required to reach the equivalence point for the strong acid than the weak acid.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
903
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos