9. Quantum Mechanics
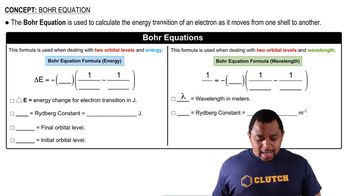
Bohr Equation
Problem 43
Textbook Question
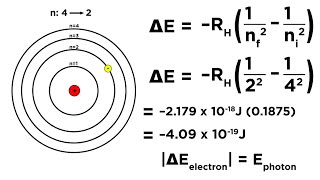
Textbook QuestionOne of the emission lines of the hydrogen atom has a wavelength of 94.974 nm. (b) Determine the initial and final values of n associated with this emission.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
1678
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos