7. Gases
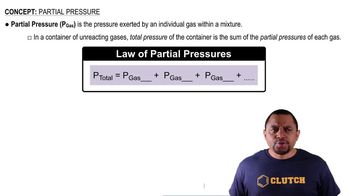
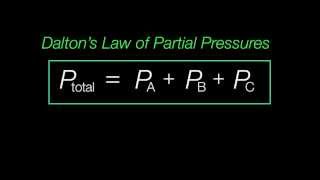
Partial Pressure
Problem 107
Textbook Question
Textbook QuestionAssume that an exhaled breath of air consists of 74.8% N2, 15.3% O2, 3.7% CO2, and 6.2% water vapor. (a) If the total pressure of the gases is 99.8 kPa, calculate the partial pressure of water vapor.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
950
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos