2. Atoms & Elements
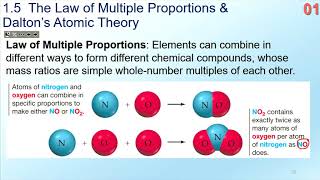
Law of Multiple Proportions

Get help from an AI Tutor
Ask a question to get started.
Problem 37
Textbook Question
Textbook QuestionSulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these are decomposed, the sulfur dioxide produces 3.49 g oxygen and 3.50 g sulfur, while the sulfur trioxide produces 6.75 g oxygen and 4.50 g sulfur. Calculate the mass of oxygen per gram of sulfur for each sample and show that these results are consistent with the law of multiple proportions.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1620
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos