7. Gases
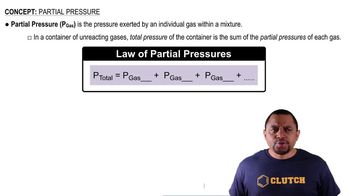
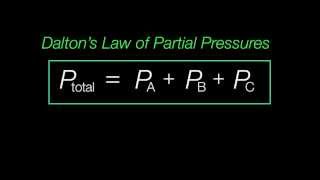
Partial Pressure
Problem 90
Textbook Question
Textbook QuestionNatural gas is a mixture of hydrocarbons, primarily methane 1CH42 and ethane 1C2H62. A typical mixture might have Xmethane = 0.915 and Xethane = 0.085. Let's assume that wehave a 15.50 g sample of natural gas in a volume of 15.00 L at a temperature of 20.00 °C. (c) What is the partial pressure of each component in the sample in atmospheres?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
459
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos