7. Gases
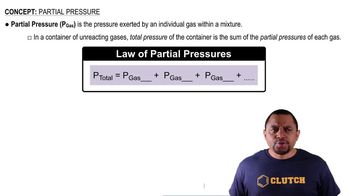
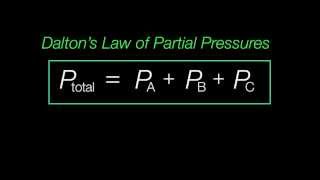
Partial Pressure
Problem 86b
Textbook Question
Textbook QuestionA mixture of 14.2 g of H2 and 36.7 g of Ar is placed in a 100.0-L container at 290 K. (a) What is the partial pressure of H2 in atmospheres?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
639
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos