18. Aqueous Equilibrium
Titrations: Strong Acid-Strong Base
Problem 104
Textbook Question
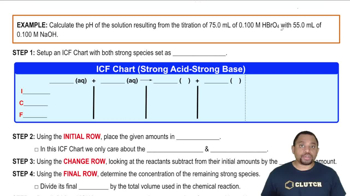
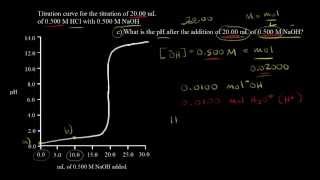
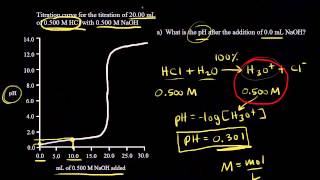
Textbook QuestionA solid sample of Fe1OH23 is added to 0.500 L of 0.250 M aqueous H2SO4. The solution that remains is still acidic. It is then titrated with 0.500 M NaOH solution, and it takes 12.5 mL of the NaOH solution to reach the equivalence point. What mass of Fe1OH23 was added to the H2SO4 solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
359
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos