3. Chemical Reactions
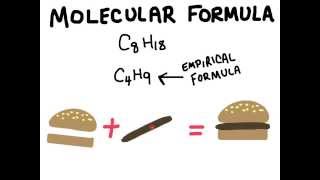
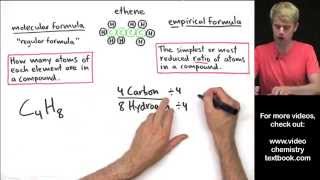
Molecular Formula
Problem 106b
Textbook Question
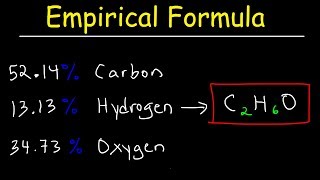
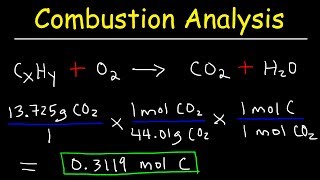
Textbook Question(b) An elemental analysis of the acid indicates that it is composed of 5.89% H, 70.6% C, and 23.5% O by mass. What is its molecular formula?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1297
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos