18. Aqueous Equilibrium
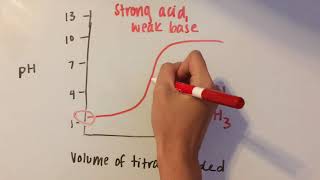
Intro to Acid-Base Titration Curves

Get help from an AI Tutor
Ask a question to get started.
Problem 90a
Textbook Question
Textbook QuestionA sample of 0.2140 g of an unknown monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.0950 M NaOH. The acid required 30.0 mL of base to reach the equivalence point. (a) What is the molar mass of the acid?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
816
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos