3. Chemical Reactions
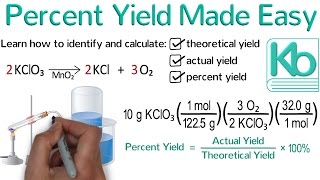
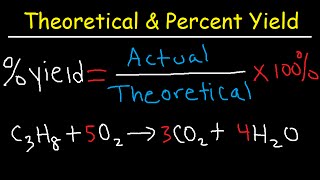
Percent Yield
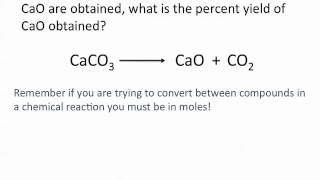
Problem 82
Textbook Question
Textbook QuestionIf 1.87 g of acetic acid (CH3COOH) reacts with 2.31 g of isopentyl alcohol (C5H12O) to give 2.96 g of isopentyl acetate (C7H14O2), what is the percent yield of the reaction?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
740
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos