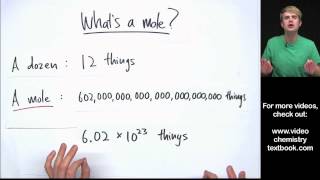2. Atoms & Elements
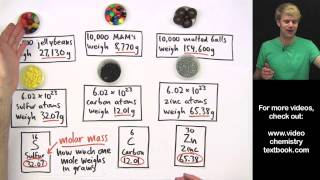
Mole Concept
Problem 41
Textbook Question
Textbook QuestionA sample of glucose, C6H12O6, contains 1.250 * 1021 carbon atoms. (c) How many moles of glucose does it contain?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
1415
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos