1. Intro to General Chemistry
Density
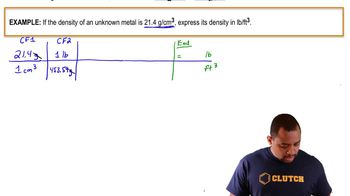
Problem 67a
Textbook Question
Textbook QuestionWhat is the density of lead in g/cm3 if a sample weighing 206.77 g has a volume of 15.50 cm3?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
924
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos