9. Quantum Mechanics
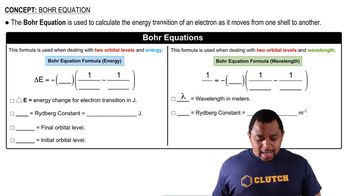
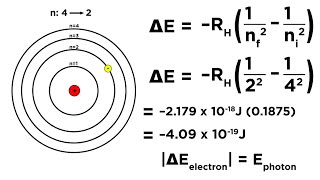
Bohr Equation
Problem 89b
Textbook Question
Textbook QuestionConsider a transition in which the electron of a hydrogen atom is excited from n = 1 to n = . (b) What is the wavelength of light that must be absorbed to accomplish this process?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
957
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos