6. Chemical Quantities & Aqueous Reactions
Molarity

Get help from an AI Tutor
Ask a question to get started.
Problem 56c
Textbook Question
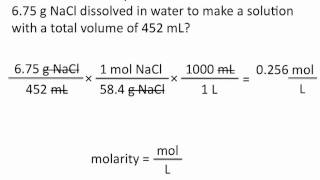
Textbook QuestionCommercial concentrated aqueous ammonia is 28% NH3 by mass and has a density of 0.90 g>mL. What is the molarity of this solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1839
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos