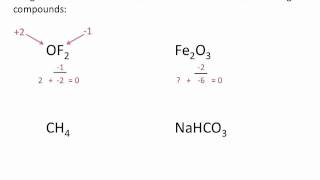6. Chemical Quantities & Aqueous Reactions
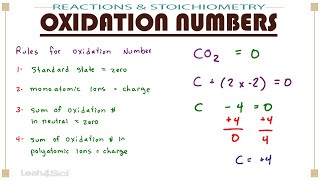
Calculate Oxidation Numbers
Problem 17
Textbook Question
Textbook QuestionFor each of the following balanced oxidation–reduction reactions, (i) identify the oxidation numbers for all the elements in the reactants and products and (ii) state the total number of electrons transferred in each reaction. (b) 2 Hg2+1aq2 + N2H41aq2 ¡ 2 Hg1l2 + N21g2 + 4 H+1aq2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
466
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos