3. Chemical Reactions
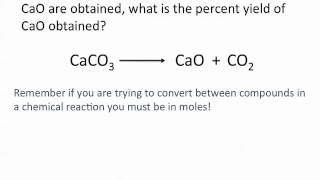
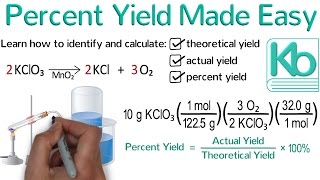
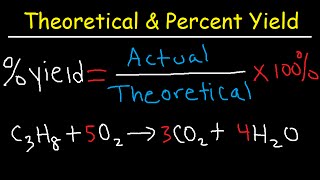
Percent Yield
Problem 83b
Textbook Question
Textbook QuestionWhen benzene 1C6H62 reacts with bromine 1Br22, bromobenzene 1C6H5Br2 is obtained: C6H6 + Br2¡C6H5Br + HBr (b) If the actual yield of bromobenzene is 42.3 g, what is the percentage yield?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
661
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos