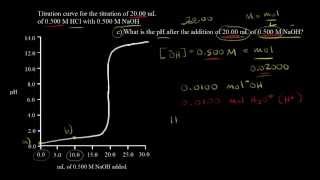
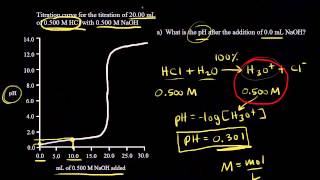
18. Aqueous Equilibrium
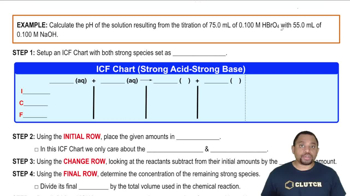
Titrations: Strong Acid-Strong Base
Problem 85b
Textbook Question
Textbook QuestionA 4.36-g sample of an unknown alkali metal hydroxide is dissolved in 100.0 mL of water. An acid–base indicator is added, and the resulting solution is titrated with 2.50 M HCl(aq) solution. The indicator changes color, signaling that the equivalence point has been reached, after 17.0 mL of the hydrochloric acid solution has been added. (b) What is the identity of the alkali metal cation: Li+, Na+, K+, Rb+, or Cs+?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
424
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos