2. Atoms & Elements
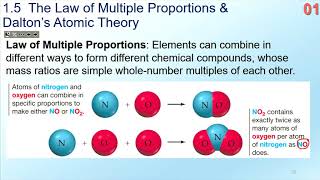
Law of Multiple Proportions

Get help from an AI Tutor
Ask a question to get started.
Problem 81
Textbook Question
Textbook QuestionIn borane, one part hydrogen combines with 3.6 parts boron by mass. A compound containing only hydrogen and boron contains 6.0 g of hydrogen and 43.2 g of boron. Could this compound be borane? If it is not borane, show that the law of multiple proportions is followed for borane and this other substance.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
483
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos