3. Chemical Reactions
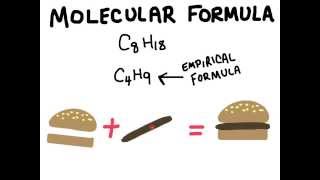
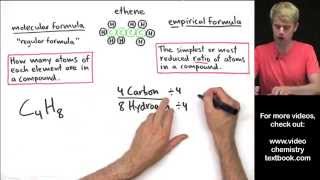
Molecular Formula
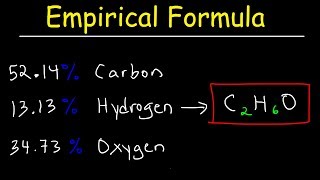
Problem 123
Textbook Question
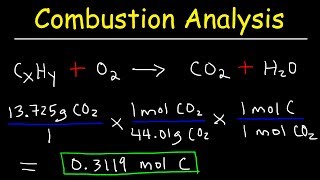
Textbook QuestionEthylene glycol, commonly used as automobile antifreeze, contains only carbon, hydrogen, and oxygen. Combustion analysis of a 23.46 mg sample yields 20.42 mg of H2O and 33.27 mg of CO2. What is the empirical formula of ethylene glycol? What is its molecular formula if it has a molecular weight of 62.0?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1125
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos