8. Thermochemistry
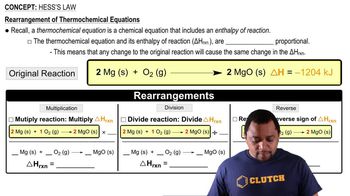
Hess's Law
Problem 104
Textbook Question
Textbook QuestionSulfuric acid (H2SO4), the most widely produced chemical in the world, is amde yb a two-step oxidaiton of sulfur to sulfur trioxide, SO3, followed by reaciton with water. Calculate ΔH°f for SO3 in kJ/mol, given the following data: S(s) + O2(g) → SO2(g) ΔH° = -296.8 kJ SO2(g) + 1/2 O2(g) → SO3(g) ΔH° = -98.9 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
893
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos