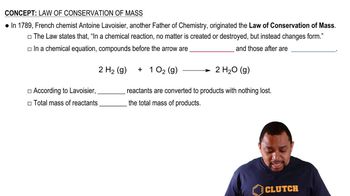
Chemical Reaction Equation
A chemical reaction equation represents the reactants and products involved in a chemical reaction, showing their respective quantities. For the decomposition of calcium carbonate (CaCO3), the balanced equation is: CaCO3(s) → CaO(s) + CO2(g). This equation is crucial for understanding the relationship between the substances involved and for calculating the mass of products formed from given reactants.

 Verified step by step guidance
Verified step by step guidance