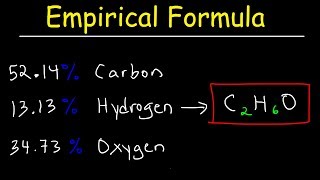
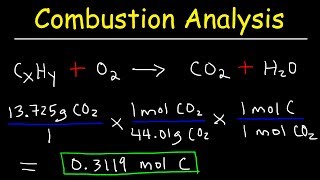
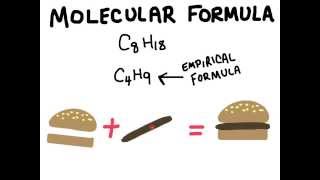
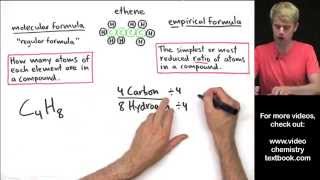
3. Chemical Reactions
Molecular Formula
Problem 92
Textbook Question
Textbook QuestionAn unknown liquid is composed of 5.57% H, 28.01% Cl, and 66.42% C. The molecular weight found by mass spectrometry is 126.58. What is the molecular formula of the compound?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
758
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos