9. Quantum Mechanics
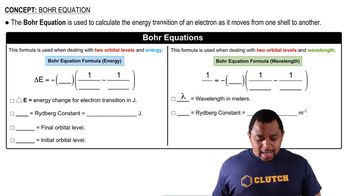
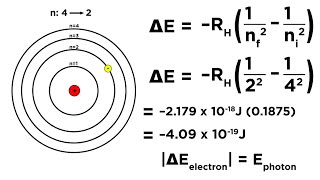
Bohr Equation
Problem 69
Textbook Question
Textbook QuestionCalculate the wavelength of the light emitted when an electron in a hydrogen atom makes each transition and indicate the region of the electromagnetic spectrum (infrared, visible, ultraviolet, etc.) where the light is found. a. n = 2¡n = 1 b. n = 3¡n = 1 c. n = 4¡n = 2 d. n = 5¡n = 2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
15mPlay a video:
1852
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos