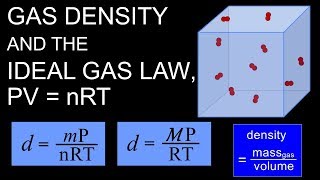
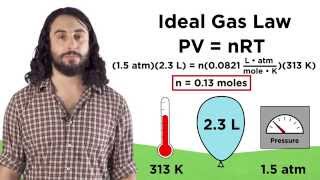
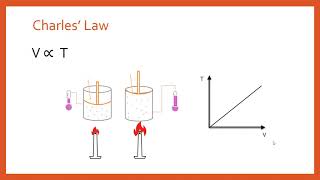
7. Gases
The Ideal Gas Law Derivations

Get help from an AI Tutor
Ask a question to get started.
Problem 4
Textbook Question
Textbook QuestionImagine that the reaction 2 CO1g2 + O21g2¡2 CO21g2 occurs in a container that has a piston that moves to maintain a constant pressure when the reaction occurs at constant temperature. Which of the following statements describes how the volume of the container changes due to the reaction: (a) the volume increases by 50%, (b) the volume increases by 33%, (c) the volume remains constant, (d) the volume decreases by 33%, (e) the volume decreases by 50%.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
454
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos