18. Aqueous Equilibrium
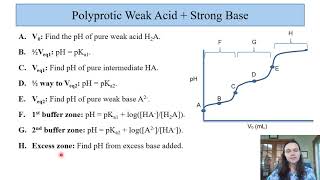
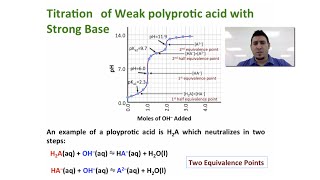
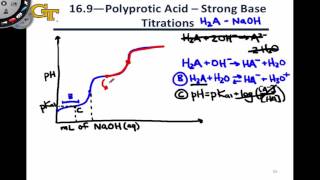
Titrations: Diprotic & Polyprotic Buffers

Get help from an AI Tutor
Ask a question to get started.
Problem 90
Textbook Question
Textbook QuestionA 30.00-mL sample of an unknown H3PO4 solution is titrated with a 0.100 M NaOH solution. The equivalence point is reached when 26.38 mL of NaOH solution is added. What is the concentration of the unknown H3PO4 solution? The neutralization reaction is H3PO4(aq) + 3 NaOH(aq)¡3 H2O(l ) + Na3PO4(aq)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
2764
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos