2. Atoms & Elements
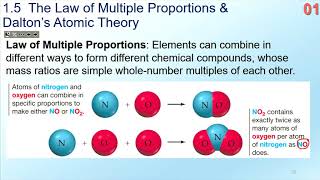
Law of Multiple Proportions
Problem 84
Textbook Question
Textbook QuestionTwo compounds containing carbon and oxygen have the following percent composition by mass. Compound 1: 42.9% carbon and 57.1% oxygen Compound 2: 27.3% carbon and 72.7% oxygen Show that the law of multiple proportions is followed. If the formula of the first compound is CO, what is the formula of the second compound?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1017
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos