When talking about thermodynamics and kinetics, one of our best friends is going to be the free energy diagram. So it's going to be essential that we learn how to interpret these correctly. The reason free energy diagrams are important is because they serve as a summary of the thermodynamics and kinetics. So first, I want to relate free energy diagrams to what we've already learned and then learn how to interpret them.
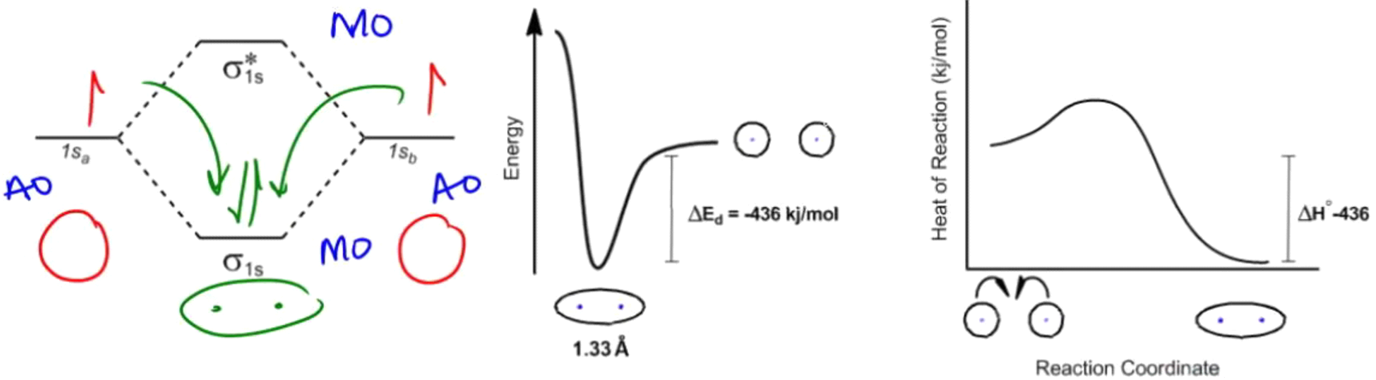
Remember that in chapter 1, maybe you were able to watch that lesson. Maybe you didn't, but it's fine. Remember that atoms save energy by forming bonds. The entire idea of a chemical bond is it's a shared region of space where they share electrons, and we could use molecular orbital diagrams to really illustrate how much energy we were saving. Remember that we had our atomic orbitals on the sides and our molecular orbitals on the top and the bottom. Even if you don't remember this that well, that's okay because I'm just going to show you that for example, for a hydrogen atom, remember that one hydrogen atom would have one electron, and another hydrogen atom would have another electron. These electrons are called 1s because they just filled a 1s orbital. This is what they would look like in terms of energy if they were not bonded to anything. Remember that by sharing electrons though, they could wind up filling their octet and their atomic orbitals.
As we said, as you make a bond between these two hydrogens, you will jump down in energy. And this is what this would look like after they're bonded because now you're saving energy by sharing those electrons. Remember that we could also use a graph of the distances to figure out how much energy we're saving as the nuclei got closer together. For example, when these hydrogen atoms, initially unbonded, get closer together, they eventually find this perfect sweet spot where they're the perfect distance apart. In this case, it was 1.33 Å, and that would be the amount of distance required to save the maximum amount of energy. The amount of energy is significant here; it just happened to be -436 kJ/mol. You don't need to memorize that at all. Later on, we're going to use a chart to figure that out.
But what is important is that all this information can be related on the free energy diagram. The free energy diagram is basically a storyline of everything that I just said. What it tells us is that this is what the atoms looked like before they were bonded. This was their energy level. After they decided to react, this is what they looked like, and now this is our energy level. So, did we save energy or did we spend energy? That's really the way we think about it. In this case, we saved energy, and it's going to be the same amount that we're talking about over here.
What we do with a free energy diagram is that the x-axis is the reaction coordinate saying as the reaction proceeds, what are the entities looking like, and then the y-axis is usually going to be either heat, talking about enthalpy, or it's going to be spontaneity, which we're going to talk about in just a second, which is the ΔG.
Free energy diagrams give us information on spontaneity and rate of reactions. What this means is that this has to do with thermodynamics and kinetics. Thermodynamics is what we call spontaneity and that describes the favorability of a reaction. So when I say that something is spontaneous, that means that it's favorable. That means that it wants to happen by itself. The equation that we use to understand thermodynamics is going to be your favorite, Gibbs free energy. We can't get away from this reaction and, I mean, from this equation. We're going to be using it pretty much for most of this chapter. Remember that it was ΔG = ΔH - TΔS. Later on, I'm going to be going in-depth on each of these variables. But for right now, just know that that's what ΔG is. It's spontaneity. ΔG is usually related by the difference in energy between the beginning and the end.
The kinetics has to do with how fast the reaction would take place if it is favorable. So, or even if it's not favorable, how fast would this reaction take place? There are a lot of very spontaneous reactions in this world that do not happen at measurable rates or appreciable rates because they're so slow. And the kinetics has to do with the activation energy that it takes in order to make a reaction go forward. So, the activation energy in the graph in the free energy diagram I gave you above would be the difference in the energy between the beginning and your highest point in the reaction. This would be my activation energy. Later on, we're going to describe that better as well. But for right now, just know that it's basically the difference between where you started and the highest point you have to achieve in order to make the reaction go forward.
So what I want to do now is I want to just do some really basic qualitative recognition here. This isn't about I don't want you guys to calculate anything yet. We're just going to decide what kind of reactions we're looking at here. Are they going to be spontaneous? Are they going to be nonspontaneous? Are they going to happen fast? Are they going to happen slow? So what I want you guys to do is go ahead and look at the following four reactions and try to figure out if it's spontaneous or not and if the rate is going to happen quickly or slowly, and then I'll go ahead and answer these for you.