11. Bonding & Molecular Structure
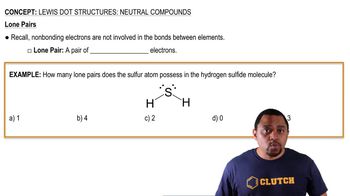
Lewis Dot Structures: Neutral Compounds
Problem 91
Textbook Question
Textbook QuestionFormic acid is responsible for the sting of ant bites. By mass, formic acid is 26.10% C, 4.38% H, and 69.52% O. The molar mass of formic acid is 46.02 g/mol. Determine the molecular formula of formic acid and draw its Lewis structure.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1863
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos