7. Gases
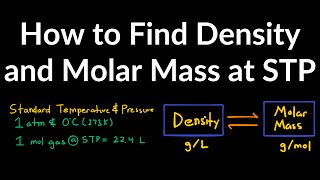
The Ideal Gas Law: Molar Mass
Problem 91
Textbook Question
Textbook QuestionGaseous compound Q contains only xenon and oxygen. When 0.100 g of Q is placed in a 50.0-mL steel vessel at 0 °C the pressure is 0.229 atm. (a) What is the molar mass of Q, and what is a likely formula?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
280
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos